Walpole MA
508-734-5893
Acton MA
978-679-1200
East Falmouth MA
508-388-7493
To schedule an appointment please call our office at (978) 679-1200 or use the patient portal
Interested? Call us now at 978-679-1200 to schedule a phone consultation to learn more!
TMS OCD Therapy
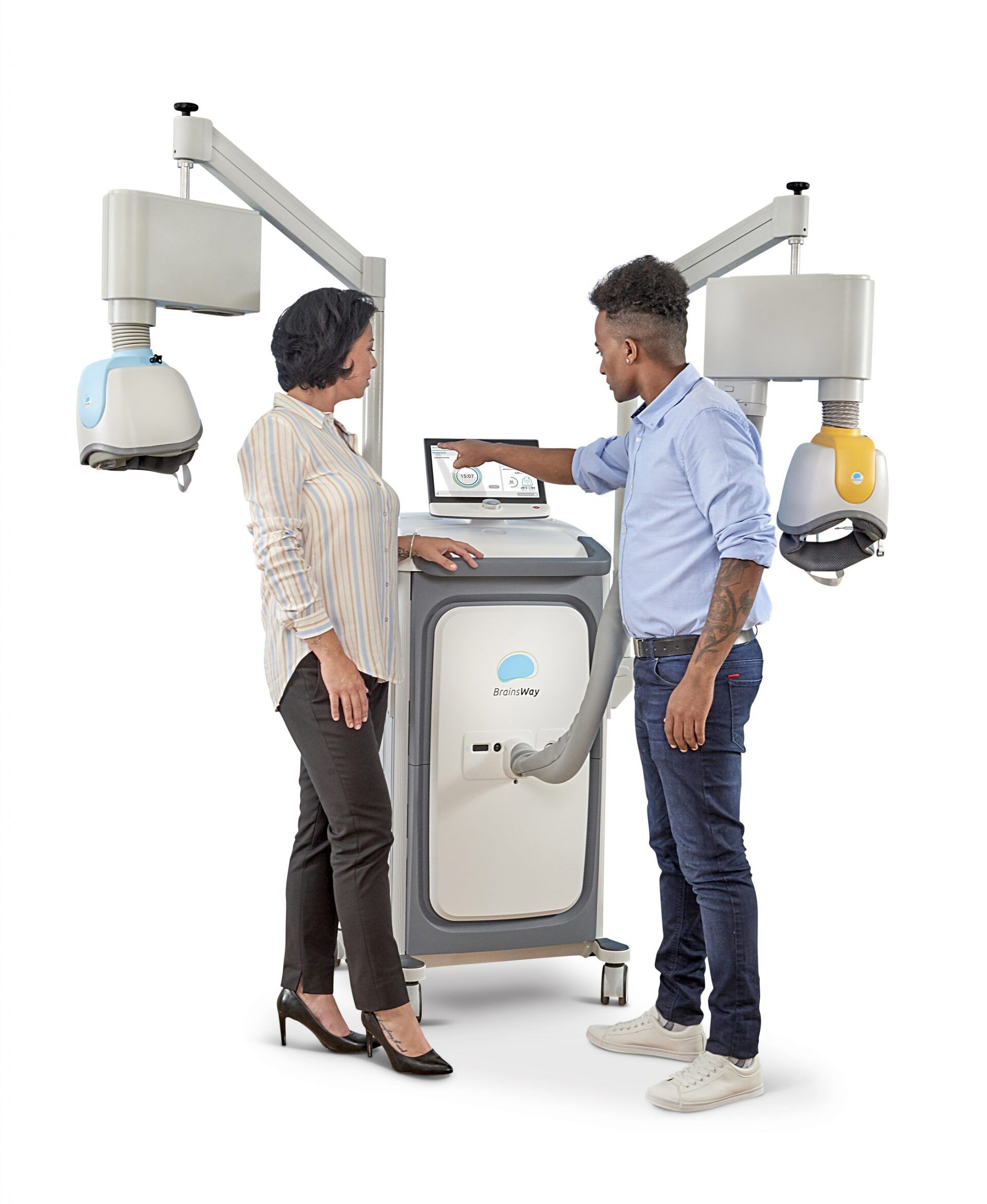
Introducing BrainsWay Deep TMS for OCD
Deep Transcranial Magnetic Stimulation, or Deep TMS™, has been shown to safely and effectively alleviate the symptoms of obsessive-compulsive disorder (OCD), particularly those patients who have not achieved sufficient improvement from traditional OCD treatment options.
The treatment utilizes a magnetic field emitted by BrainsWay’s patented H7-coil to directly reach broader and deeper brain regions than its predecessors, regulating the neural activity of brain structures associated with OCD – specifically the anterior cingulate cortex and medial prefrontal cortex.
A peer-reviewed multicenter clinical study found Deep TMS to be a highly effective OCD treatment, with more than one in three treatment-resistant OCD patients achieving “response”, greatly improving their quality of life.
As a noninvasive procedure, Deep TMS is a well-tolerated treatment that does not cause any adverse or long-lasting side effects. It does not require a significant recovery period, and the 18-min treatment can easily be integrated into each patient’s day-to-day schedule.
Treatment Options in Your Battle Against OCD
Deep TMS: The first noninvasive device FDA-cleared to treat OCD, Deep TMS utilizes magnetic fields to safely regulate the neural activity of brain structures found to be related to OCD. A multicenter study published in 2019 by the American Journal of Psychiatry confirmed the treatment’s efficacy, concluding that focusing brain stimulation on “the medial prefrontal cortex and anterior cingulate cortex significantly improved OCD symptoms.” Deep TMS is also safe to combine with other forms of therapy, and does not cause any adverse or long-lasting side effects. It is noninvasive and can be easily incorporated into the patient’s daily schedule.
Cognitive Behavioral Therapy: CBT is a form of psychotherapy commonly used to treat OCD. The treatment focuses on the thoughts, feelings, behavior and physical reactions linked to OCD, while helping patients become less anxious in reaction to them. One subtype, Acceptance and Commitment Therapy (ACT), assists patients in developing openness and flexibility when reacting to obsessive thoughts, while committing to behavioral change.
Exposure and Response Prevention Therapy: ERP has also been shown to be a form of psychotherapy effective in treating OCD. ERP gradually exposes the patient to the source of their anxiety, accompanied by the support of a mental health professional, who encourages them to refrain from acting on their compulsions.
Psychopharmacology: Medication for OCD is also considered among the treatment options found helpful in reducing OCD symptoms. Several antidepressants have been FDA-approved to treat OCD, including selective serotonin reuptake inhibitors (SSRIs) and one tricyclic antidepressant (TCA). While many patients find them to be helpful, these medications can also induce possible side effects, such as weight gain and sexual dysfunction.
Lifestyle Changes to Protect Against OCD: Healthy eating, regular exercise and “sleep hygiene” (eliminating distractions when going to bed) are seen as a “winning triad” that greatly affects our ability to ward off OCD symptoms. Additional protective factors include keeping up with your regularly scheduled activities and incorporating stress management techniques such as meditation, yoga, and massage therapy into your life.
- How effective is BrainsWay?
In real-life clinical settings[2], the treatment was proven to be effective for 3 out of 4 patients, with a 51% remission rate and 75% response rate.
- I have already tried other medications and had poor results – will BrainsWay be any better?
Studies have shown the treatment to be effective for patients with depression, no matter how many other medications were tried in the current depressive episode.
- Has BrainsWay been tested?
BrainsWay uses Deep TMS technology, which has been tested in over 60 clinical studies for various indications in leading institutions worldwide. The FDA has cleared this technology for clinical use in Major Depressive Disorder and it is currently available in the US, Europe and South America[3]. Over 15,000 patients have already been treated with BrainsWay.
- How does the treatment work?
The treatment integrates seamlessly into your routine. During each session, you are comfortably seated on a chair and a cushioned helmet is placed over your head. The helmet uses magnetic fields to stimulate the targeted brain area and improve depressive symptoms. The procedure requires daily sessions of 20 minutes for 4-6 weeks. As the treatment does not require hospitalization or anesthesia, and entails no memory loss and no systemic side effects, you can return to your normal routine after each session.
- Does the treatment entail any discomfort?
BrainsWay is a non-invasive procedure, which eliminates the need for hospitalization. Patients may experience minor side effects such as headaches or minor pain at the site of the treatment. However, this is usually brief and ceases after he first few sessions.
[1] Safety information: Patients should consult with their doctor before undergoing BrainsWay. The most common side effects include headaches and application site pain or discomfort. There is also a very rare risk of seizure associated with the treatment. Patients with metal in or around the head such as in metal plates, implants and stents, should not undergo Deep TMS treatment.
[2] Remission Response Rates: In the multicenter trial that led to BrainsWay’s FDA clearance for MDD, 1 in 3 medication-resistant patients achieved full remission at 38.4% achieved a positive response. Real life settings have shown significantly better results. Data on file with BrainsWay
[3] Indication: BrainsWay is indicated by the FDA for the treatment of depressive episodes in adult patients suffering from Major Depressive Disorder, who failed to achieve satisfactory improvement from previous anti-depressant medication treatment in the current episode. FDA 510(k) No. K122288.
Our Locations:
New England Center for Healthy Minds
289 Great Road, Suite G1
Acton, MA 01720
CapeTMS
178 Teaticket Hwy Unit 4
East Falmouth MA 02536
Walpole TMS
1600 Providence Hwy, #145
Walpole, Ma 02081
Office Hours
Monday : 9:00 am - 5:00 pm
Tuesday : 9:00 am - 5:00 pm
Wednesday : 9:00 am - 5:00 pm
Thursday : 9:00 am - 5:00 pm
Friday : 9:00 am - 3:00 pm
New England Center for Healthy Minds, All Rights Reserved 2021 All data and information provided on this site is for informational purposes only. New England Center for Healthy Minds & Baystate Websites and Marketing LLC makes no representations as to accuracy, completeness, correctness, suitability, or validity of any information on this site and will not be liable for any errors, omissions, or delays in this information or any losses, injuries, or damages arising from its display or use. All information is provided on an as-is basis.
In Acton Contact us
Fax: (978) 274-2032
In Falmouth Contact Us
In Walpole Contact us
Contact us
Fax: (508) 660-7493