Walpole MA
508-734-5893
Acton MA
978-679-1200
East Falmouth MA
508-388-7493
To schedule an appointment please call our office at (978) 679-1200 or use the patient portal
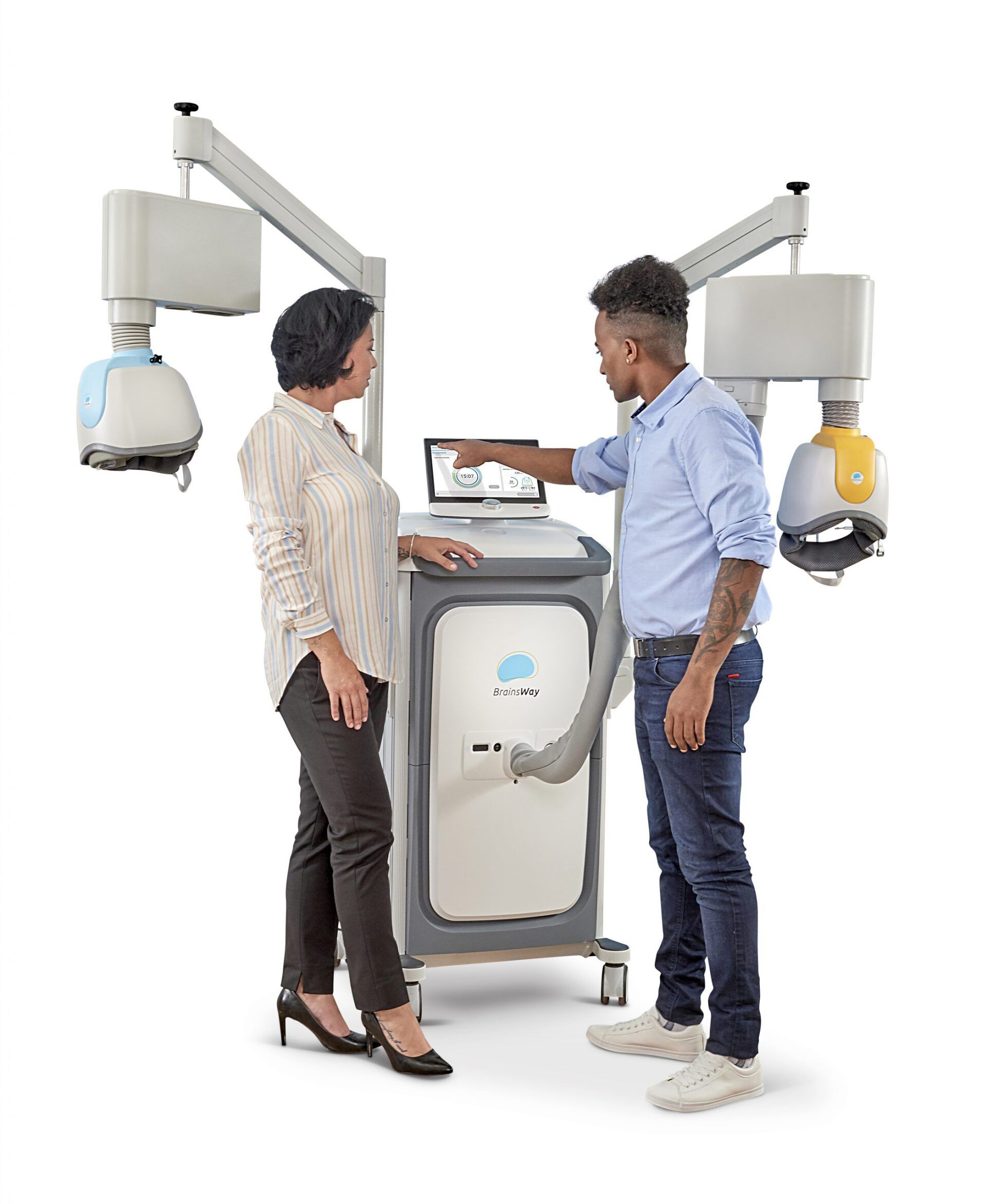
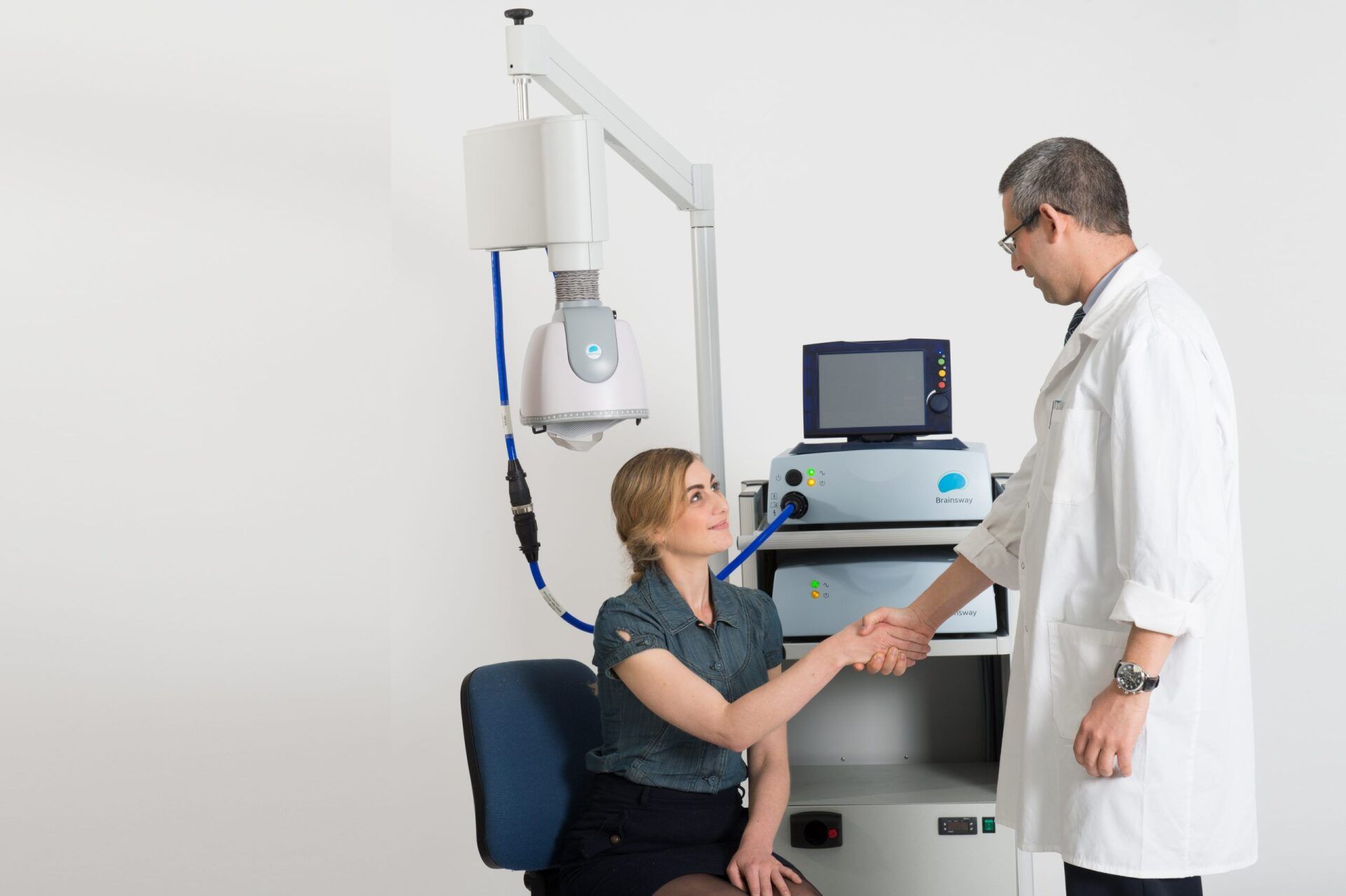
TMS Therapy
BrainsWay: An effective treatment for depression that is quick, well tolerated and non-invasive.
If you’ve been diagnosed with major depressive disorder (MDD) and one or more antidepressant medications haven’t worked for you, BrainsWay can help you achieve wellbeing.
With each therapy session lasting approximately 20 minutes, the treatment can fit easily into your schedule. The treatment has minimal side effects[1] and is covered by Medicare and most private payers.
Interested? Call us now at 978-679-1200 to schedule a phone consultation to learn more!
- How effective is BrainsWay?
In real-life clinical settings[2], the treatment was proven to be effective for 3 out of 4 patients, with a 51% remission rate and 75% response rate.
- I have already tried other medications and had poor results – will BrainsWay be any better?
Studies have shown the treatment to be effective for patients with depression, no matter how many other medications were tried in the current depressive episode.
- Has BrainsWay been tested?
BrainsWay uses Deep TMS technology, which has been tested in over 60 clinical studies for various indications in leading institutions worldwide. The FDA has cleared this technology for clinical use in Major Depressive Disorder and it is currently available in the US, Europe and South America[3]. Over 15,000 patients have already been treated with BrainsWay.
- How does the treatment work?
The treatment integrates seamlessly into your routine. During each session, you are comfortably seated on a chair and a cushioned helmet is placed over your head. The helmet uses magnetic fields to stimulate the targeted brain area and improve depressive symptoms. The procedure requires daily sessions of 20 minutes for 4-6 weeks. As the treatment does not require hospitalization or anesthesia, and entails no memory loss and no systemic side effects, you can return to your normal routine after each session.
- Does the treatment entail any discomfort?
BrainsWay is a non-invasive procedure, which eliminates the need for hospitalization. Patients may experience minor side effects such as headaches or minor pain at the site of the treatment. However, this is usually brief and ceases after he first few sessions.
[1] Safety information: Patients should consult with their doctor before undergoing BrainsWay. The most common side effects include headaches and application site pain or discomfort. There is also a very rare risk of seizure associated with the treatment. Patients with metal in or around the head such as in metal plates, implants and stents, should not undergo Deep TMS treatment.
[2] Remission Response Rates: In the multicenter trial that led to BrainsWay’s FDA clearance for MDD, 1 in 3 medication-resistant patients achieved full remission at 38.4% achieved a positive response. Real life settings have shown significantly better results. Data on file with BrainsWay
[3] Indication: BrainsWay is indicated by the FDA for the treatment of depressive episodes in adult patients suffering from Major Depressive Disorder, who failed to achieve satisfactory improvement from previous anti-depressant medication treatment in the current episode. FDA 510(k) No. K122288.
Our Locations:
New England Center for Healthy Minds
289 Great Road, Suite G1
Acton, MA 01720
CapeTMS
178 Teaticket Hwy Unit 4
East Falmouth MA 02536
Walpole TMS
1600 Providence Hwy, #145
Walpole, Ma 02081
Office Hours
Monday : 9:00 am - 5:00 pm
Tuesday : 9:00 am - 5:00 pm
Wednesday : 9:00 am - 5:00 pm
Thursday : 9:00 am - 5:00 pm
Friday : 9:00 am - 3:00 pm
New England Center for Healthy Minds, All Rights Reserved 2021 All data and information provided on this site is for informational purposes only. New England Center for Healthy Minds & Baystate Websites and Marketing LLC makes no representations as to accuracy, completeness, correctness, suitability, or validity of any information on this site and will not be liable for any errors, omissions, or delays in this information or any losses, injuries, or damages arising from its display or use. All information is provided on an as-is basis.
In Acton Contact us
Fax: (978) 486-4037
In Falmouth Contact Us
Office 508-388-7493
Fax 774-612-3026
In Walpole
Contact us
Fax: (508) 660-7493